
The time scale for events related to proteins. NMR-based techniques
Price: $ 44.50
4.9(377)
Download scientific diagram | The time scale for events related to proteins. NMR-based techniques appropriate for studying dynamics at different time scales are shown in boxes. from publication: Protein structure and dynamics | Proteins are essential components of biological processes, this explains why understanding their structure, function and dynamics is so important. In the following, we give an overview on various methods for the determination of three-dimensional structure and dynamics of | Protein Structure, Molecular Graphics and Graphical Model | ResearchGate, the professional network for scientists.

A Simple Method To Predict Protein Flexibility Using Secondary Chemical Shifts
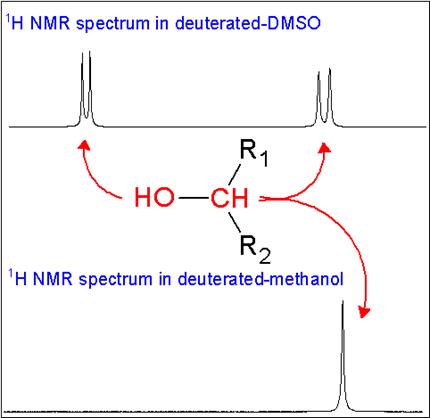
Exchangeable Protons in NMR—Friend or Foe? - ACD/Labs

Functional protein dynamics on uncharted time scales detected by nanoparticle-assisted NMR spin relaxation

Timescale of protein dynamic events, and the appropriate experimental
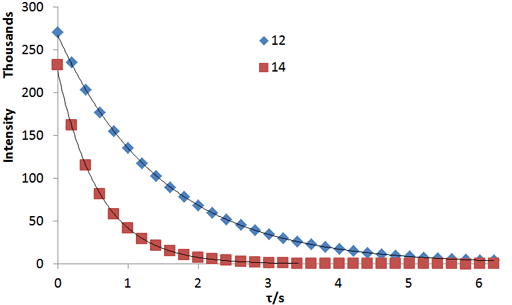
NMR Relaxation

Automated Glycan Assembly of 19F‐labeled Glycan Probes Enables High‐Throughput NMR Studies of Protein–Glycan Interactions - Fittolani - 2021 - Angewandte Chemie International Edition - Wiley Online Library

Cross-linking mass spectrometry discovers, evaluates, and corroborates structures and protein–protein interactions in the human cell

Affinity measurement of strong ligands with NMR spectroscopy: Limitations and ways to overcome them - ScienceDirect

In-Cell Structural Biology by NMR: The Benefits of the Atomic Scale
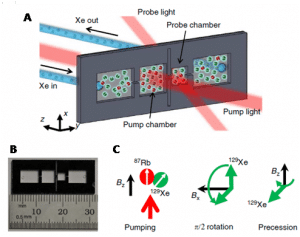
NMR active nuclei and solid state NMR Biomedical

Protein Analysis Techniques Explained - ATA Scientific
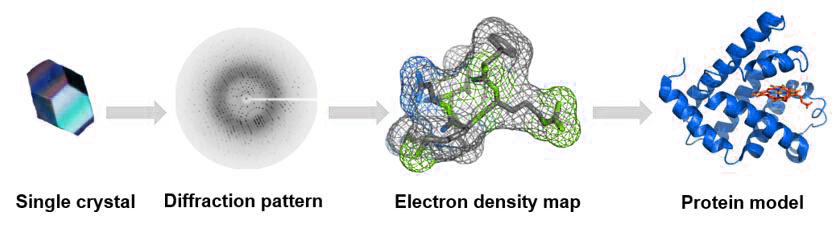
Comparison of Crystallography, NMR and EM - Creative Biostructure

Measurement of Ligand–Target Residence Times by 1H Relaxation Dispersion NMR Spectroscopy




