
NH2OH (A)H2SO4 (B). Product B is: -OH NH NH NH NH
Price: $ 35.50
4.5(599)
Click here:point_up_2:to get an answer to your question :writing_hand:nh2ohah2so4 b product b isohnhnhnhnh.
Click here👆to get an answer to your question ✍️ NH2OH -A-H2SO4 -B- Product B is- -OH NH NH NH NH
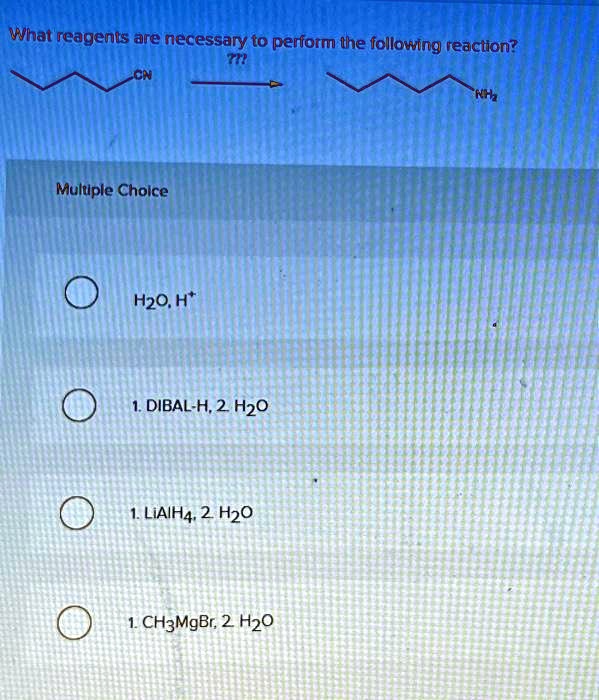
SOLVED: please help thanks! What reagents are necessary to perform the following reaction? TTP CN NH Multiple Choice HO,H* 1.DIBAL-H,2.H20 1.LiAIH4.2HO 1.CH3MgBr.2.H20

56. What will be the product obtained after polymerisation of X in given reaction? O 02 LU O - (i)NH2OH (ii) H2SO4 (b) Nylon 5 (9) Nylon 4 (d) Nylon 4,6 (a) Nylon 6

Challenging the Limits of Nitro Groups Associated with a Tetrazole Ring

Sequential Pd(0)/Fe(III) Catalyzed Azide–Isocyanide Coupling/Cyclization Reaction: One-Pot Synthesis of Aminotetrazoles

39) H2/ Ni F (CH3)2 NH - NO2 DMF, A. (X) (Y), X & Y. H2NOH A H2SO4 B , Identify A and B.

Sulfur-promoted, one-pot, and metal-free conversion of aromatic aldehydes to nitriles using an inorganic ammonium salt as the nitrogen source - ScienceDirect

Sulfur-promoted, one-pot, and metal-free conversion of aromatic aldehydes to nitriles using an inorganic ammonium salt as the nitrogen source - ScienceDirect
19798-80-2, 4-Chloropyridin-2-amine

PDF) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space




