
FDA Clears Nanowear's SimpleSense Non-Invasive Continuous Blood
Price: $ 20.50
4.7(132)
Nanowear's remote monitoring device and its SimpleSense platform received FDA 510(k) clearance as a continuous blood pressure monitor.
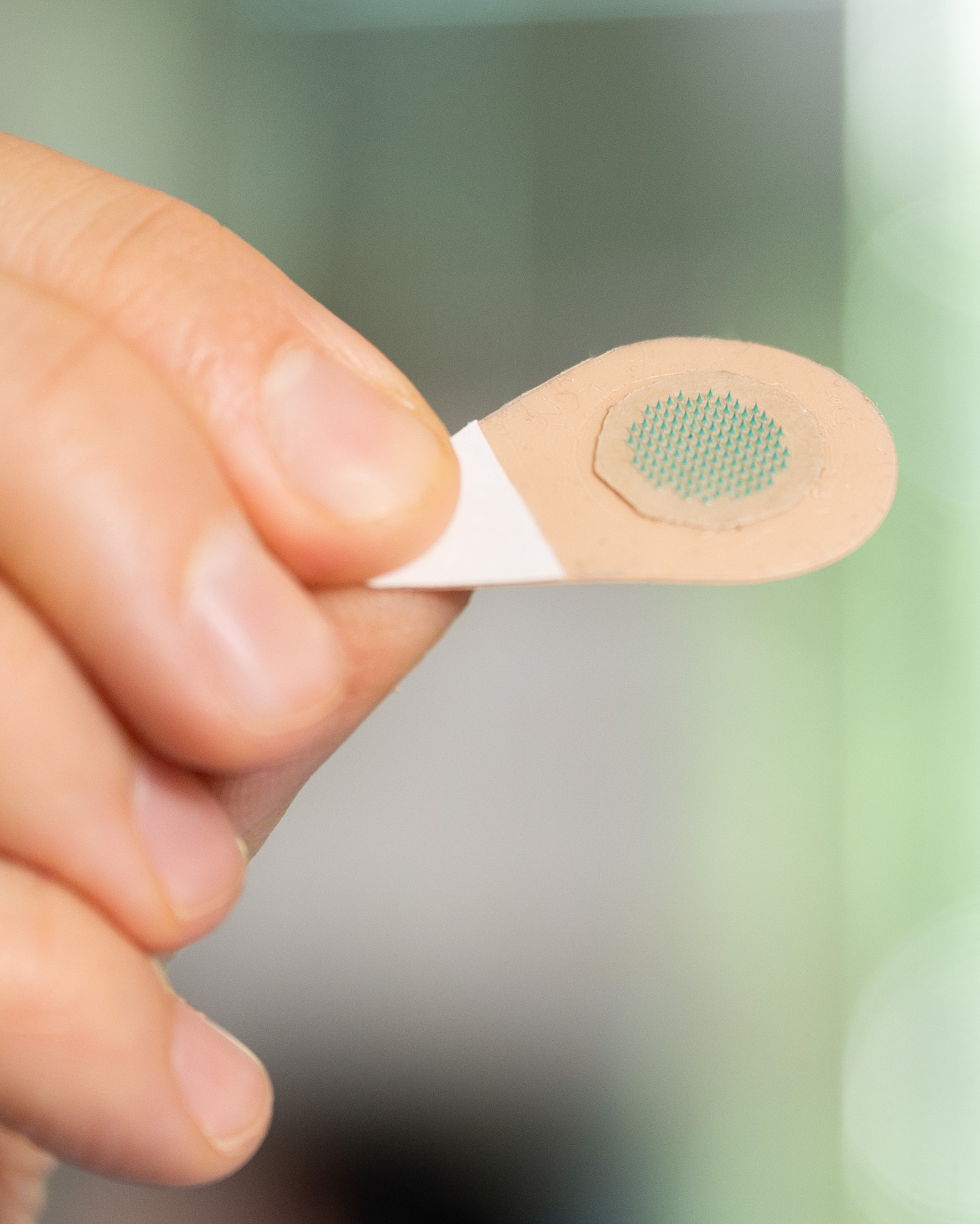
Microneedle Tattoos Encode Complex Health Information

Nanowear gets FDA clearance for undergarment that estimates blood pressure
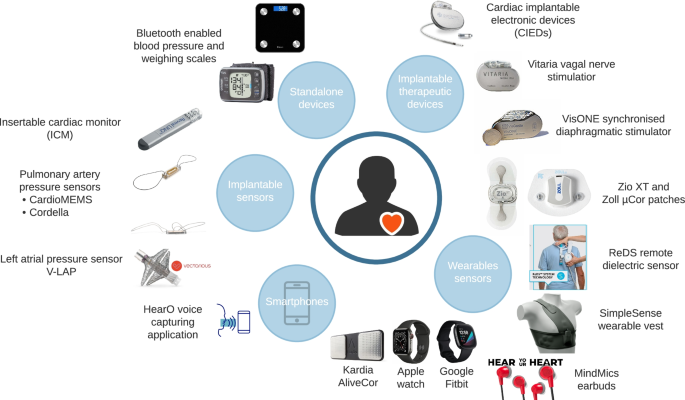
Digital Technologies to Support Better Outcome and Experience of Care in Patients with Heart Failure

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous Blood Pressure Monitoring and Hypertension Diagnostic Management: SimpleSense-BP, Business & Finance

Nanowear Announces FDA 510(k) Clearance for AI-enabled Continuous Blood Pressure Monitoring and Hypertension Diagnostic Management: SimpleSense-BP
Precision Medicine Patient Care Articles & News - Inside Precision Medicine

Inside Precision Medicine
Precision Medicine Precision Medicine Articles & News - Inside Precision Medicine

FDA OKs Nihon Kohden's Ventilator System

FDA 510(k) clearance for SimpleSense-BP

Nanowear wins FDA nod for wearable blood pressure monitor
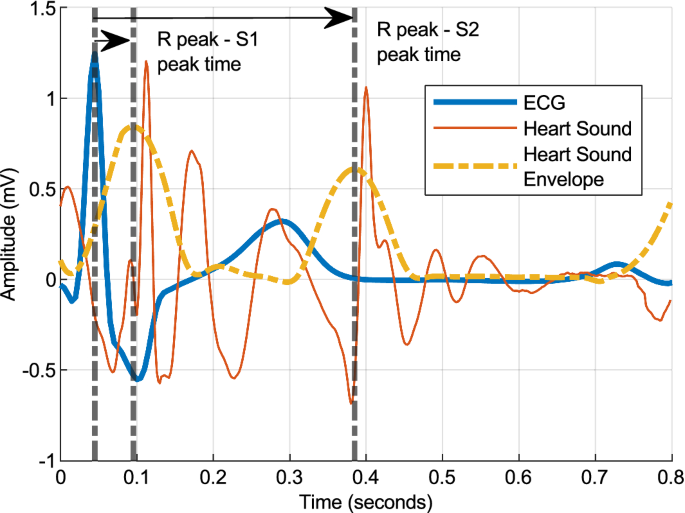
Multiparametric cloth-based wearable, SimpleSense, estimates blood pressure

AdvaMed (@AdvaMedUpdate) / X
:max_bytes(150000):strip_icc()/Libbey_Ascent16PieceAssortedGlasswareSet_04-edba90d2c1b0440b937de3027db2f6d1.jpg)



